Potassium is the 19th element of the periodic table and one of the alkali metals. Potassium has very similar properties to sodium (Na), those elements in the same Group, and close in mass as well.
1. Potassium is a soft metal that can be cut even with a knife. Freshly cut potassium is silvery in appearance, but in the air it immediately begins to tarnish toward gray.
2. The symbol for potassium on the periodic table is “K,” derived from the Neo-Latin word “kalium,” which in turn came from the Arabic “al-qalyah,” meaning plant ashes.
3. Potassium is the seventh most abundant metal that makes up about 2.4 percent of the mass of Earth’s crust, including billions of tons of potassium chloride, according to the Royal Society of Chemistry.
4. Potassium is less dense than water and equals – 0.8629 g/cm3.
5. Potassium is an extremely chemically active metal and therefore it does not occur in nature in pure form. It reacts violently even with air, which is why it is usually stored under mineral oil, such as kerosene.
6. When potassium reacts with water, it produces hydrogen gas and heat so rapidly that the hydrogen often ignites, creating a distinctive lilac flame.
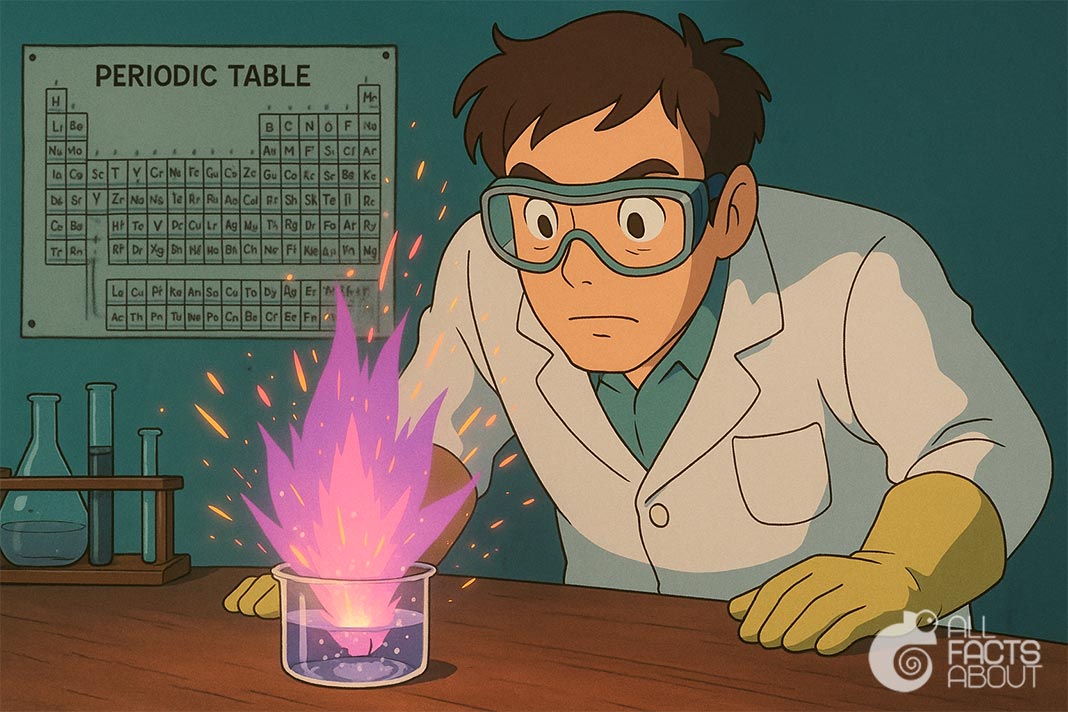
Chemical Reaction of Potassium in Water.
8. Like all alkali metals, potassium easily melts (melting point 63.51 °C) and begins to evaporate even with relatively low temperature (boiling point of potassium 761 °C).
9. Potassium hydroxide is used in manufacturing soaps and detergents, ceramics and glass, and textile dyes. Potassium bromide is used chiefly in photography, photoengraving, and medicines.
10. Potassium is an essential element in the human body; it is responsible for water and salt balance in cells and involved in the work of internal organs and muscles.
11. Most of the total body potassium is inside the cells and the next largest proportion is in the bones.
12. Potassium is a macronutrient as well as calcium, sodium, and magnesium. In water it dissolves into ions and helps to conduct electrical impulses in the body. If there is a deficiency of potassium in the body, the work of our nervous system is disrupted. Low potassium leads to weakness and lack of energy.
13. The recommended daily potassium intake for children ranges from 600 to 1700 milligrams, for adults – from 1800 to 5000 milligrams. The need for potassium depends on body weight, physical activity, physiological condition, and even the climate.
14. Potassium helps the cells function properly. A balanced amount of this element is very important for our health. Good sources of potassium: seafood, bananas, chocolate, avocados, potatoes, parsley, nuts, and tomatoes.




